Richard Smalley, 1943-2005
Richard Smalley shared the 1996 Nobel Prize in Chemistry with Robert Curl and Harold Kroto for the discovery of buckminsterfullerene, a molecule made from 60 carbon atoms. It was named after Buckmister Fuller, an American architect and inventor who designed the geodesic dome, a structure that looks like the fullerene molecule.

The discovery of buckyballs was significant for many reasons, but a few stand out. First, it represented a new allotrope of carbon (allotrope is a fancy word for the for structural forms an element can assume). Before this, only graphite and diamond were known allotropes. Since then, nanotubes have been discovered, as have higher fullerenes with many more carbon atoms. At the time, however, it wasn't known that elemental carbon could be so diverse.
Second, with the exquisite symmetry of the molecule, the properties were expected to be remarkable, and they are.
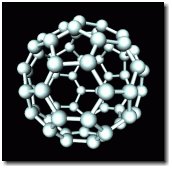
Finally, discovering that carbon could form into perfect little sphere-like molecules started people thinking seriously about nanotechnology.
Generally, the discovery of a single molecule gets little press. With the discovery of buckminsterfullerene, Professor Smalley was instrumental in the touching off of a fertile new area of scientific exploration.